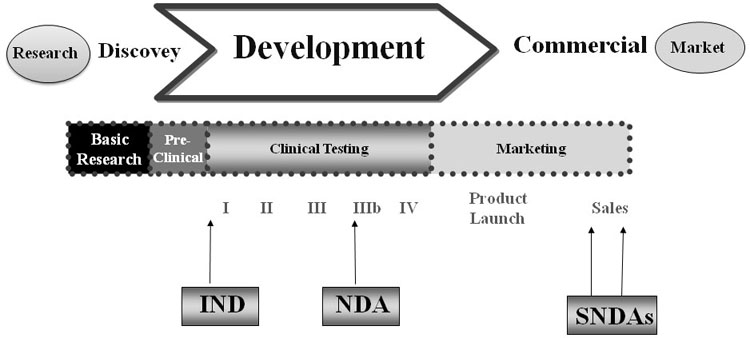
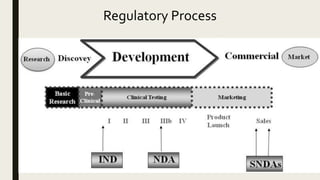
Book 8: 2023 Clinical Research Dictionary & Introduction to the FDA Dr – Clinical Research Resources, LLC
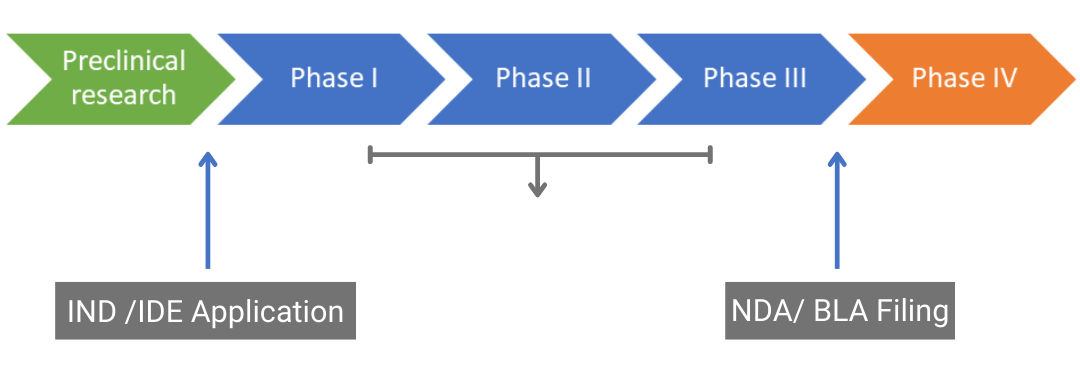
Drug approval pathway. IND: Investigational New Drug, IDE: Investigational Device Exemption, NDA: New Drug Application, BLA: Biologics License Application - ProRelix Research







![InD] Capstone Research Integrated Designpreneurship Final Presentation InD] Capstone Research Integrated Designpreneurship Final Presentation](https://www.polyu.edu.hk/sd/sd/-/media/department/sd/events/2022/12/indfinalpresentationedmv420221117.jpg?bc=ffffff&h=630&w=1200&hash=DDA2779449E749C8A1317EB6C6A6A1DE)